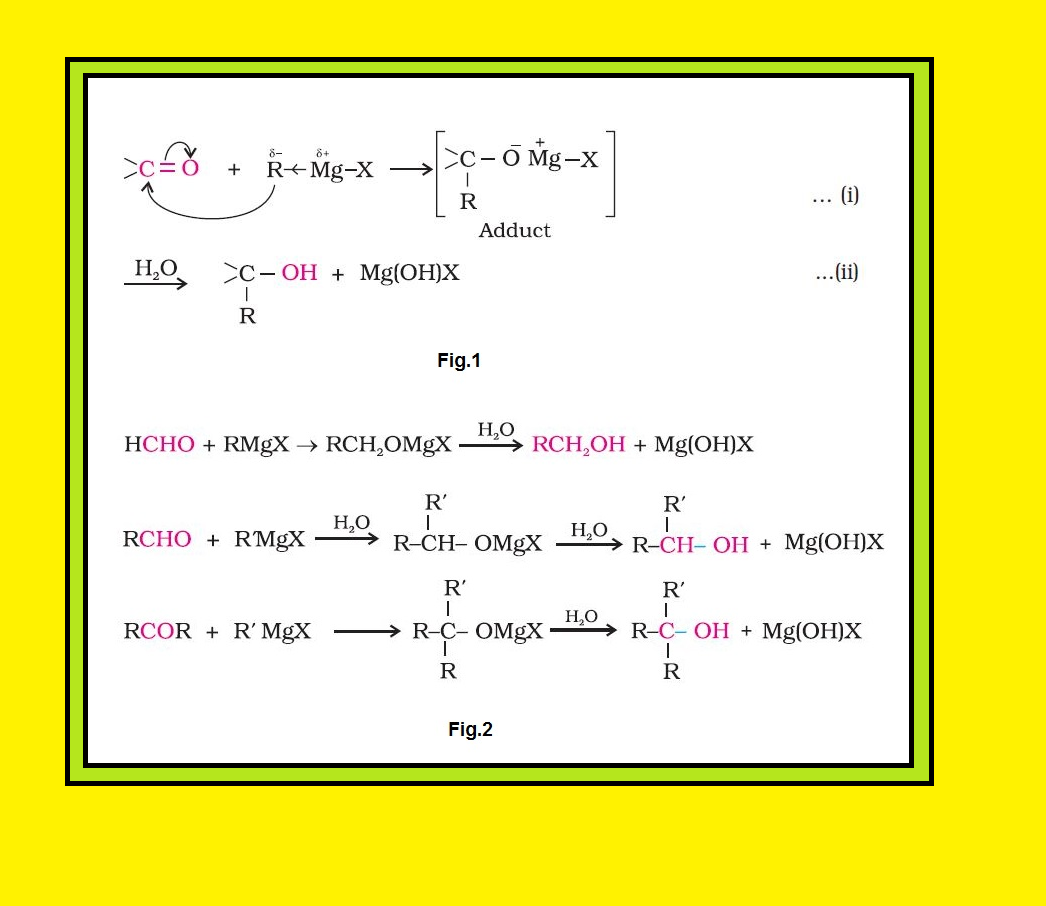
From alkenes :

(i) `color{green}(text(By acid catalysed hydration ))` : Alkenes react with water in the presence of acid as catalyst to form alcohols. In case of unsymmetrical alkenes, the addition reaction takes place in accordance with Markovnikov’s rule. See fig.1 for overall mechanism.
(ii) `color{green}(text(By hydroboration–oxidation ))` : Diborane `color{red}(BH_3)_2` reacts with alkenes to give trialkyl boranes as addition product. This is oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide. See fig.2.
● The addition of borane to the double bond takes place in such a manner that the boron atom gets attached to the `color{red}(sp^2)` carbon carrying greater number of hydrogen atoms.
● The alcohol so formed looks as if it has been formed by the addition of water to the alkene in a way opposite to the Markovnikov’s rule. In this reaction, alcohol is obtained in excellent yield.
(ii) `color{green}(text(By hydroboration–oxidation ))` : Diborane `color{red}(BH_3)_2` reacts with alkenes to give trialkyl boranes as addition product. This is oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide. See fig.2.
● The addition of borane to the double bond takes place in such a manner that the boron atom gets attached to the `color{red}(sp^2)` carbon carrying greater number of hydrogen atoms.
● The alcohol so formed looks as if it has been formed by the addition of water to the alkene in a way opposite to the Markovnikov’s rule. In this reaction, alcohol is obtained in excellent yield.